Atmospheric composition and climate/Description: Difference between revisions
Jump to navigation
Jump to search
m (Text replace - "C cycle and natural vegetation dynamics" to "Natural vegetation and carbon cycle") |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ComponentDescriptionTemplate | {{ComponentDescriptionTemplate | ||
|Status=On hold | |Status=On hold | ||
|Reference= | |Reference= Forster et al., 2007; WMO/UNEP, 2013; | ||
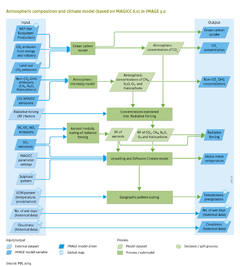
|Description=The IMAGE climate model calculates atmospheric CO2 concentration levels, on the basis of CO2 emission data for energy, industry and land-use change ([[Emissions]]), terrestrial carbon balance ([[Natural vegetation and carbon cycle]]), and carbon uptake by the oceans (calculated in [[MAGICC model|MAGICC]] on the basis of the [[Bern Ocean Carbon model]]). For other long-lived greenhouse gases (CH4, N2O, and halocarbons), and ozone (O3) precursors (CO, NMVOC), MAGICC calculates the resulting emissions via a simple atmospheric chemistry module. Halocarbons and N2O concentrations mostly show a simple mass-concentration conversion and half-life behaviour. CH4 and ozone dynamics are more complex, with CH4 lifetime depending on the OH concentration level, and both O3 and OH concentration levels depending on CH4, NOx, CO and NMVOC emissions ([[Meinshausen et al., 2011b]]). | |Description=The IMAGE climate model calculates atmospheric CO2 concentration levels, on the basis of CO2 emission data for energy, industry and land-use change ([[Emissions]]), terrestrial carbon balance ([[Natural vegetation and carbon cycle]]), and carbon uptake by the oceans (calculated in [[MAGICC model|MAGICC]] on the basis of the [[Bern Ocean Carbon model]]). For other long-lived greenhouse gases (CH4, N2O, and halocarbons), and ozone (O3) precursors (CO, NMVOC), MAGICC calculates the resulting emissions via a simple atmospheric chemistry module. Halocarbons and N2O concentrations mostly show a simple mass-concentration conversion and half-life behaviour. CH4 and ozone dynamics are more complex, with CH4 lifetime depending on the OH concentration level, and both O3 and OH concentration levels depending on CH4, NOx, CO and NMVOC emissions ([[Meinshausen et al., 2011b]]). | ||
A change in atmospheric gas concentrations also changes the amount of radiation absorbed or transmitted by the atmosphere, and thereby causes a change in the earth’s energy balance and temperature. This change in the energy balance is expressed as ‘radiative forcing’ per gas, measured in W/m2. In MAGICC, concentrations of long-lived greenhouse gases and O3 are translated into radiative forcing values using the estimates from the IPCC's AR4 ([[Forster et al., 2007]]). | A change in atmospheric gas concentrations also changes the amount of radiation absorbed or transmitted by the atmosphere, and thereby causes a change in the earth’s energy balance and temperature. This change in the energy balance is expressed as ‘radiative forcing’ per gas, measured in W/m2. In MAGICC, concentrations of long-lived greenhouse gases and O3 are translated into radiative forcing values using the estimates from the IPCC's AR4 ([[Forster et al., 2007]]). | ||
Revision as of 16:29, 16 December 2013
Parts of Atmospheric composition and climate/Description
| Component is implemented in: |
|
| Related IMAGE components |
| Projects/Applications |
| Models/Databases |
| Key publications |
| References |