Nutrients: Difference between revisions
Jump to navigation
Jump to search
No edit summary |
No edit summary |
||
| Line 6: | Line 6: | ||
|InputVar=Population; GDP per capita; Land cover, land use - grid; Fertilizer use efficiency; Animal stock; Livestock ration; Manure spreading fraction; Nitrogen deposition - grid; NH3 loss; Fraction of urban population; Actual crop and grass production - grid; | |InputVar=Population; GDP per capita; Land cover, land use - grid; Fertilizer use efficiency; Animal stock; Livestock ration; Manure spreading fraction; Nitrogen deposition - grid; NH3 loss; Fraction of urban population; Actual crop and grass production - grid; | ||
|OutputVar=NH3 emission - grid; N and P discharge to surface water; Nutrient discharge to water surface; Soil N budget - grid; Soil P budget - grid; | |OutputVar=NH3 emission - grid; N and P discharge to surface water; Nutrient discharge to water surface; Soil N budget - grid; Soil P budget - grid; | ||
|Parameter=Fraction NH3 loss; | |Parameter=Fraction NH3 loss; | ||
|Description=Human | |Description=Human activity has accelerated the Earth’s biogeochemical nitrogen (N) and phosphorus (P) cycles through increasing fertiliser use in agriculture ([[Bouwman et al., 2011]]). Increased use of N and P fertilisers has raised food production to support the rapidly growing world population, and increasing per capita consumption particularly of meat and milk ([[Galloway et al., 2004]]). Increased fertiliser use has contributed to ongoing increases in crop yields. | ||
This has resulted in | A side effect is that significant proportions of the mobilised N are lost through ambient emissions of ammonia (NH3), nitrous oxide (N2O) and nitric oxide (NO). Ammonia contributes to eutrophication and acidification when deposited on land. Nitric oxide plays a role in tropospheric ozone chemistry, and nitrous oxide is a potent greenhouse gas. Moreover, large proportions of mobilised N and P in watersheds enter the groundwater through leaching, and are released to surface waters through groundwater transport and surface runoff. Subsequently, nutrients in streams and rivers are transported to coastal marine systems, reduced by retention but augmented by releases from point sources, such as sewerage systems and industrial facilities. | ||
To assess eutrophication as a consequence of | This has resulted in negative impacts on human health and the environment, such as groundwater pollution, loss of habitat and biodiversity, an increases in the frequency and severity of harmful algal blooms, eutrophication, hypoxia and fish kills ([[Diaz and Rosenberg, 2008]]; [[Zhang et al., 2010]]). The harmful effects of eutrophication have spread rapidly around the world, with large-scale implications for biodiversity, water quality, fisheries and recreation, in both industrialised and developing regions ([[UNEP, 2002]]). Input of nutrients in freshwater and coastal marine ecosystems, also disturbs the stoichiometric balance of N, P and silica (Si) ([[Rabalais, 2002]]) affecting total plant production and the species composition in ecosystems. | ||
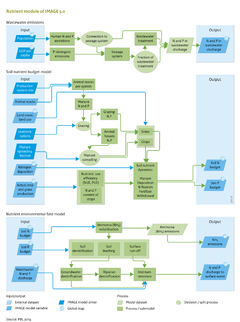
# | To assess eutrophication as a consequence of increasing population, and economic and technological development, IMAGE 3.0 includes a nutrient model, which comprises three sub-models: | ||
# | # Wastewater emissions to generate nutrient flows in wastewater discharges (See figure on the right I); | ||
# Soil nutrient budget to describe all input and output of N and P in soil compartments (See figure on the right II); | |||
# Nutrient environmental fate to describe the fate of soil nutrient surpluses and wastewater nutrients in the aquatic environment, (See figure on the right III). | |||
|ComponentCode=N | |ComponentCode=N | ||
|FrameworkElementType=state component | |FrameworkElementType=state component | ||
}} | }} | ||
Revision as of 10:48, 12 February 2014
| Component is implemented in: |
| Components: |
| Related IMAGE components |
| Projects/Applications |
| Key publications |
| References |
Key policy issues
- How will the increasing use of fertilisers affect terrestrial and marine ecosystems, with possible consequences for human health?
- To what extent can the negative impacts be reduced by more efficient nutrient management and wastewater treatment, while retaining the positive effects on food production and land productivity?